Researchers Face Potential Danger from Protein Particles in the Lab
Proper cleaning of laboratory equipment reduces contamination and protects Parkinson's disease researchers
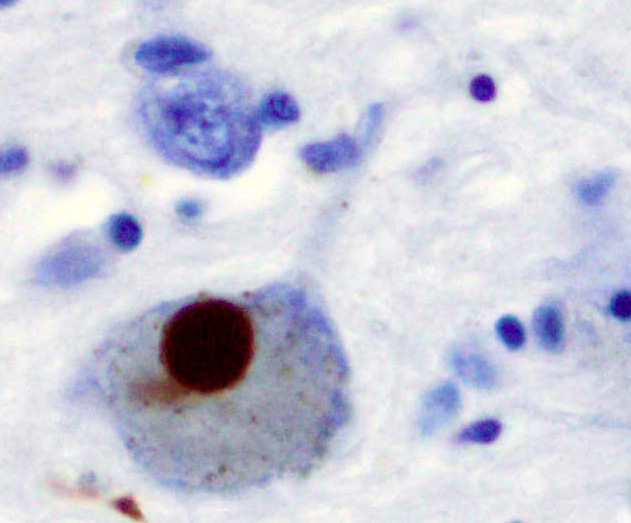 A Lewy body (stained brown) in a brain cell of the substantia nigra in Parkinson's disease.Source: wikipediaLewy bodies and Lewy neurites are found in the brains of Parkinson’s disease (PD) patients. They consist primarily of fibrils of the protein alpha-synuclein (α-Syn), which self-assembles into fibrils in vitro. If introduced into the human body, these seeds can act as prions and trigger the formation of toxic protein deposits. Because α-Syn fibrils are often used in research, it is important that they are not accidentally transferred to humans or cell cultures. Researchers reporting in the Journal of Parkinson’s Disease describe three cleaning procedures that effectively remove and disassemble these α-synuclein seeds.
A Lewy body (stained brown) in a brain cell of the substantia nigra in Parkinson's disease.Source: wikipediaLewy bodies and Lewy neurites are found in the brains of Parkinson’s disease (PD) patients. They consist primarily of fibrils of the protein alpha-synuclein (α-Syn), which self-assembles into fibrils in vitro. If introduced into the human body, these seeds can act as prions and trigger the formation of toxic protein deposits. Because α-Syn fibrils are often used in research, it is important that they are not accidentally transferred to humans or cell cultures. Researchers reporting in the Journal of Parkinson’s Disease describe three cleaning procedures that effectively remove and disassemble these α-synuclein seeds.
α-Syn is purified and assembled in test tubes into fibrils that are used to investigate/mimic PD pathogenesis in model animals ranging from worms (c. elegans) to rodents and non-human primates in a large number of laboratories. These laboratories typically contain surfaces and non-disposable items made from plastic, glass, aluminum, or stainless steel. These items are often rough, with areas that cannot be completely cleaned by wiping. Therefore, it is important to minimize contamination through effective cleaning procedures.
“Several teams, including ours, demonstrated that fibrillar α-Syn propagate from one cell, including neurons, to another and amplify during this propagation process mimicking prion particle behavior. These observations suggest that fibrillar α-Syn is not innocuous,” explained lead investigator Ronald Melki, PhD, Director of Research at the Paris-Saclay Institute of Neurosciences, CNRS, Gif-sur-Yvette, France.
Researchers applied a solution of fluorescently-labeled fibrils and ribbons of α-Syn to roughened surfaces mimicking laboratory conditions. The droplets were easily visible to the eye and could be assessed by their fluorescence.
Five cleaning solutions were tested: 1) sodium hypochlorite (20,000 ppm); 2) sodium hydroxide (1N), 3) sodium dodecyl sulfate (SDS, 1%, W/V); 4) Hellmanex (1%, V/V); and 5) TFD4 (1%, V/V). As a control, the surfaces were washed with just commercially prepared pure water. After cleaning, the amount of small assemblies, fibrils, and ribbons of α-Syn remaining on the surfaces was measured by fluorescence. To evaluate whether the α-Syn fibrils that were washed off the surfaces were destroyed, fibrils and ribbons were incubated in the various cleaning solutions for one hour and the amount of remaining fibrils was measured.
The researchers found that the commercial detergents Hellmanex, and SDS (1%, W/V) are the most suitable cleaning reagents for removal and neutralization of α -Syn seeds from contaminated surfaces. However, solutions of sodium hypochlorite (20,000 ppm) or sodium hydroxide (1N), previously shown to diminish prion infectivity, were ineffective. Plain water was similarly ineffective.
As the result of this investigation, the research teams have implemented “Laboratory Standard Operating Procedures for fibrillar α-Syn” to minimize possible contamination. This includes a cleaning procedure that removes and disassembles α-Syn fibrils adsorbed on plastic, glass, aluminum, or stainless steel surfaces as well as recommended disposal procedures for various forms of α-Syn waste. This cleaning method is sufficiently mild to allow efficient decontamination of non-disposable tools in a laboratory.
“We conclude that cleaning procedures relying on the use of detergents that are compatible with most non-disposable tools in a laboratory are simple to implement and highly recommended when working with fibrillar α-Syn in a laboratory setting,” stated co-investigator Patrik Brundin, MD, PhD, Editor-in-Chief of the Journal of Parkinson’s Disease and Director of the Center for Neurodegenerative Science at Van Andel Research Institute in Grand Rapids, Michigan.
“The procedures we describe remove and inactivate α-Syn fibrillar assemblies to a level where they are undetectable, which significantly improve researchers’ safety when handling fibrillar α-Syn. Further work is needed to establish the infectious unit of recombinant α-Syn and the biological efficiency of the cleaning,” added co-investigator Luc Bousset, PhD, at the Paris-Saclay Institute of Neurosciences, CNRS.

